You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Anode Question
- Thread starter JSM
- Start date
JSM
Member III
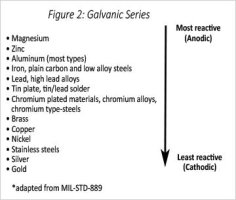
Thanks , I'm in fresh water and was under the impression I had to use magnesium.Magnesium is at the very top of the chart for most reactive metals (ahead of zinc and aluminum). It will give the most protection but will sacrifice itself most quickly. Most use aluminum or zinc "zincs."
View attachment 42353
We are in fresh water, and have all been advised to change from zinc to aluminum, for the last few years. (but not all the way to Magnesium)Thanks , I'm in fresh water and was under the impression I had to use magnesium.
Since there's zero chance of getting to salt or brackish water where I am, I went with magnesium on my last anode change based on what anode manufacturer, Martyr, says.Thanks , I'm in fresh water and was under the impression I had to use magnesium.
I'm new to this world, but I've understood that ground faults on your boat (or others in your marina), in addition to being dangerous for swimmers, could cause faster-than-expected deterioration. Someone please correct me if I have it wrong.Someone suggested that my shore power may be the culprit. Anyone have any suggestions as to where I can begin checking?
peaman
Sustaining Member
I believe that installation of a galvanic isolator on the boat's ground wire will provide protection both for the boat and for those swimmers. Pretty easy to install, since it only involves the ground (green) wire at the on-board shore power connection.I've understood that ground faults on your boat (or others in your marina), in addition to being dangerous for swimmers, could cause faster-than-expected deterioration.
Avoiding the bulk, the weight and expense of an isolation transformer, I installed a galvanic isolator some years ago. Better than nothing, according to my electrician friends, but an isolation transformer is still the "gold standard".I believe that installation of a galvanic isolator on the boat's ground wire will provide protection both for the boat and for those swimmers. Pretty easy to install, since it only involves the ground (green) wire at the on-board shore power connection.
Last edited:
fstbttms
Professional Hull Diver
Thanks , I'm in fresh water and was under the impression I had to use magnesium.
Use magnesium anodes in freshwater. Zinc is for sure a no-no and aluminum will work, but magnesium is best.
Ralph Hewitt
Member III
What about moored out in Salt water... no shore power?Use magnesium anodes in freshwater. Zinc is for sure a no-no and aluminum will work, but magnesium is best.
fstbttms
Professional Hull Diver
Zinc or aluminum, shorepower or no.What about moored out in Salt water... no shore power?
What about moored out in Salt water... no shore power?
my boat is moored in brackish water. this season i plan to use 2 anodes - 1 lead + 1 aluminum. Overkill, adds weight, ... but I'm curious and want to try it
fstbttms
Professional Hull Diver
my boat is moored in brackish water. this season i plan to use 2 anodes - 1 lead + 1 aluminum. Overkill, adds weight, ... but I'm curious and want to try it
That's a very bad idea. Mixing anode materials creates a battery at worst and at best leads to rapid depletion of one of the anodes. In brackish water you should be using aluminum exclusively.
BTW- Lead is not used for sacrificial anodes. Zinc is.
Jerry VB
E32-3 / M-25XP
Trivia: Lead is actually more noble than steel and iron - the steel that the lead is connected to will etch away, "protecting" the lead. (This is a problem with lead ballast in metal boats if the ballast is not properly isolated from the hull.)BTW- Lead is not used for sacrificial anodes. Zinc is.
I discovered this with an older car with a side pole battery. The threads of the bolts that attached the leads to the battery were eaten away and I had to replace the bolts. The threads in the battery were fine. I first thought the battery threads in the "soft" lead were stripped out, but it was actually the "hard" steel bolts that had gone bad.
Ref: https://www.mfcp.com/technical-info/galvanic-corrosion
Sorry I meant to type Zinc. I didn't realize that mixing aluminum and zinc would have caused an issue. Is zinc bad in brackish waters ?That's a very bad idea. Mixing anode materials creates a battery at worst and at best leads to rapid depletion of one of the anodes. In brackish water you should be using aluminum exclusively.
BTW- Lead is not used for sacrificial anodes. Zinc is.
fstbttms
Professional Hull Diver
Sorry I meant to type Zinc. I didn't realize that mixing aluminum and zinc would have caused an issue. Is zinc bad in brackish waters ?
Zinc is not "bad" in brackish water, it just doesn't provide the protection your boat needs in brackish water.